I spent the winter of 2017/18 doing a research internship in Dr. Dongwhan Lee’s lab at Seoul National University.
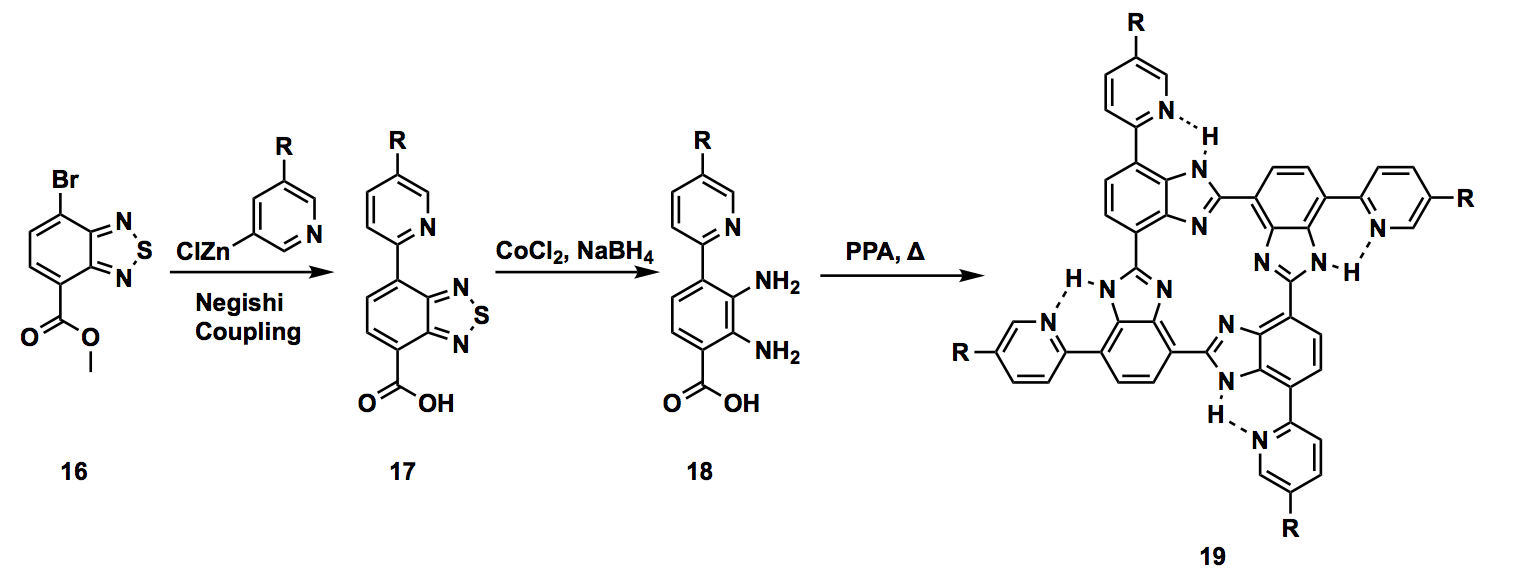
The project I got involved focused on designing organometallic catalysis. Especially, our target molecule, cyclic tetrabenzimidazole, had a distinct characteristic that the electronic environment of its central metal can be modulated by the secondary coordination sphere. Modulating the secondary coordination sphere is loosely defined as the utilizing non-covalent interactions, not the atoms directly connected to the central metal, to change the chemical activity including reactivity, selectivity, and functions. However, it is a challenge to utilize non-covalent interactions, which are relatively weak and usually non-directional, in a reliable and reproducible manner. Moreover, preventing unwanted multimetallic species is critical to assure the catalysis’s chemical functions. Key to solve these issues can be found in biological molecules. Cyclic tetrabenzimidazole was also bio-inspired in the sense that the direct environment of the center resembles the one of a porphyrin, the ligand component of Heme, with even more rigid structure. Organic syntheses involve multiple steps and usually faced by numerous failures. My project was also full of troubleshooting. Specifically, acquisition of pyridyl halide having an alkyl chain to increase the solubility of the molecule was challenging. I independently devised two different routes to obtain this moiety using Sonogashira cross-coupling reaction. I managed to successfully synthesize this part of the molecule by the end of the internship. Although I did not involve to the end of the total synthesis, I certainly contributed the part of the challenges in the synthetic route.